
Legiolert*
For detection of Legionella pneumophila



- Legionella test for all serotypes of Legionella pneumophila with equal or greater accuracy than traditional spread plate culture methods.1-12
- Significantly improves Legionella testing workflow, leading to higher productivity.
- Liquid culture test; isolates can be stored and/or serotyped.
- Reports in MPN, equivalent to CFU per ISO 6107:2021.
- Published by ASTM International as Standard D8429-21.
- Received NF Validation by AFNOR Certification, reference number IDX 33/06-06/19.
Overview
Easy
- Improved Legionella testing workflow for increased laboratory profitability.
- Less than 5 minutes of hands-on time compared to 10-40 minutes required for traditional spread plate methods.13
- Objective and easy-to-read results.
- No confirmation step required.
Accurate
- More resistant to competing non-Legionella organisms than traditional spread plate methods.1,2,7-9
- Demonstrated to be as or more sensitive than traditional spread plate methods in several independent, peer-reviewed studies. 1-4,6-12
- Highly specific for all serotypes of Legionella pneumophila, confirmed in several peer-reviewed studies.1-3,8-10,12
Accepted
- Used by public, utility, and private laboratories around the world.
- Published by ASTM International as Standard D8429-21.
- Received NF Validation by AFNOR Certification, reference number IDX 33/06-06/19.
- Listed as bacterial enzyme culture method in ASHRAE Guideline 12.
- Included in the UK Standing Committee of Analysts’ Blue Book.
Why test for Legionella pneumophila?
- Causes 100% of deaths from Legionnaires’ disease outbreaks14-19
- Causes 97% of Legionnaires’ disease cases, based on data from cultures of 4,719 patients over seven years in 17 countries20
- The only cause of Legionnaires’ disease cooling tower outbreaks17-21
- Saves time and resources; eliminates unnecessary remediations for non-pneumophila species
Science
How the Legiolert Test works
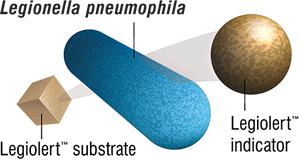
The Legiolert Test detects Legionella pneumophila in water samples. This test is based on a bacterial enzyme detection technology that signals the presence of Legionella pneumophila through utilization of a substrate present in the Legiolert reagent. Legionella pneumophila cells grow rapidly and reproduce using the rich supply of amino acids, vitamins, and other nutrients present in the Legiolert reagent. Actively growing strains of Legionella pneumophila use the added substrate to produce a brown color indicator. The Legiolert Test detects Legionella pneumophila at 1 organism in 100 mL within 7 days.
How to use
Learn how to use the Legiolert test
Potable protocols
Potable (10mL protocol)
Potable (100mL protocol)
Nonpotable protocols
Nonpotable (1.0mL protocol)

